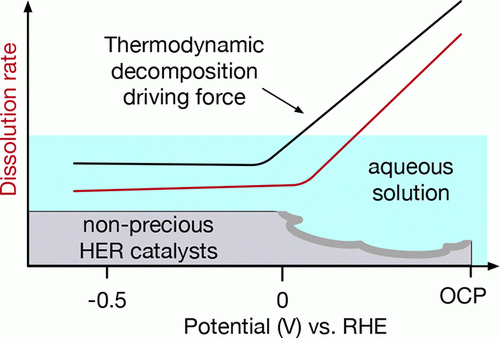
Nonprecious hydrogen evolution reaction (HER) catalysts commonly suffer from severe dissolution under open-circuit potential (OCP). In this work, using calculated Pourbaix diagrams, we quantitatively analyze the stability of a set of well-known active HER catalysts (MoS2, MoP, CoP, Pt in acid, and Ni3Mo in base) under working conditions. We determine that the large thermodynamic driving force toward decomposition created by the electrode/electrolyte interface potential is responsible for the substantial dissolution of nonprecious HER catalysts at OCP. Our analysis further shows the stability of HER catalysts in acidic solution is ordered as Pt ≈ MoS2 > MoP > CoP, which is confirmed by the measured dissolution rates using an inductively coupled plasma mass spectrometer. On the basis of the gained insights, we suggest strategies to circumvent the catalyst dissolution in aqueous solution. READ MORE