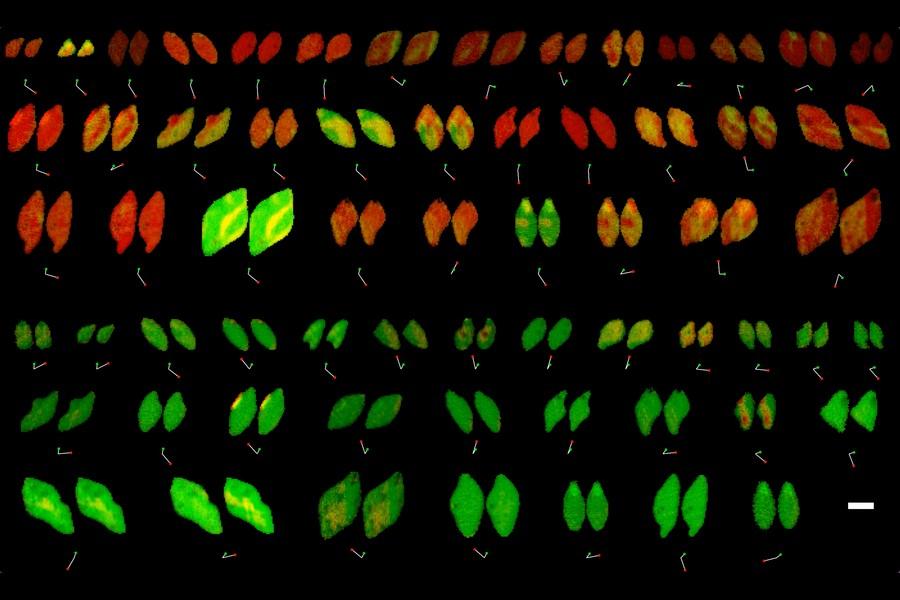
Reaction rates at spatially heterogeneous, unstable interfaces are notoriously difficult to quantify, yet are essential in engineering many chemical systems, such as batteries1 and electrocatalysts2. Experimental characterizations of such materials by operando microscopy produce rich image datasets3,4,5,6, but data-driven methods to learn physics from these images are still lacking because of the complex coupling of reaction kinetics, surface chemistry and phase separation7. Here we show that heterogeneous reaction kinetics can be learned from in situ scanning transmission X-ray microscopy (STXM) images of carbon-coated lithium iron phosphate (LFP) nanoparticles. Combining a large dataset of STXM images with a thermodynamically consistent electrochemical phase-field model, partial differential equation (PDE)-constrained optimization and uncertainty quantification, we extract the free-energy landscape and reaction kinetics and verify their consistency with theoretical models. We also simultaneously learn the spatial heterogeneity of the reaction rate, which closely matches the carbon-coating thickness profiles obtained through Auger electron microscopy (AEM). Across 180,000 image pixels, the mean discrepancy with the learned model is remarkably small (<7%) and comparable with experimental noise. Our results open the possibility of learning nonequilibrium material properties beyond the reach of traditional experimental methods and offer a new non-destructive technique for characterizing and optimizing heterogeneous reactive surfaces. READ MORE